UH Researcher Solving an Age-Old Mystery about Crystal Formation
Incorporation of Molecules Occurs in Two Steps, Divided by an Intermediate State

A million years ago, the oldest known species to walk upright like a human, the Homo erectus, had a human-like fascination with crystals. Historians can even pin down the possible reasons – crystals didn’t look like anything around at the time - trees, valleys, mountains. Crystals were a material to ponder, a fascinating diversion for the mind.
To this day, the human preoccupation with the magic of crystals continues to fill the mind’s eye of scientists who have developed ways to use crystals for everything from malaria cures to solar cells and semiconductors, catalysts and optical elements. Over the years crystals have become crucial constituents of the technologies that enable modern civilization.

Humans have been fascinated by crystals for many millennia, from the time of Homo erectus to the Renaissance and beyond.
Humans have been fascinated by crystals for many millennia, from the time of Homo erectus to the Renaissance and beyond.
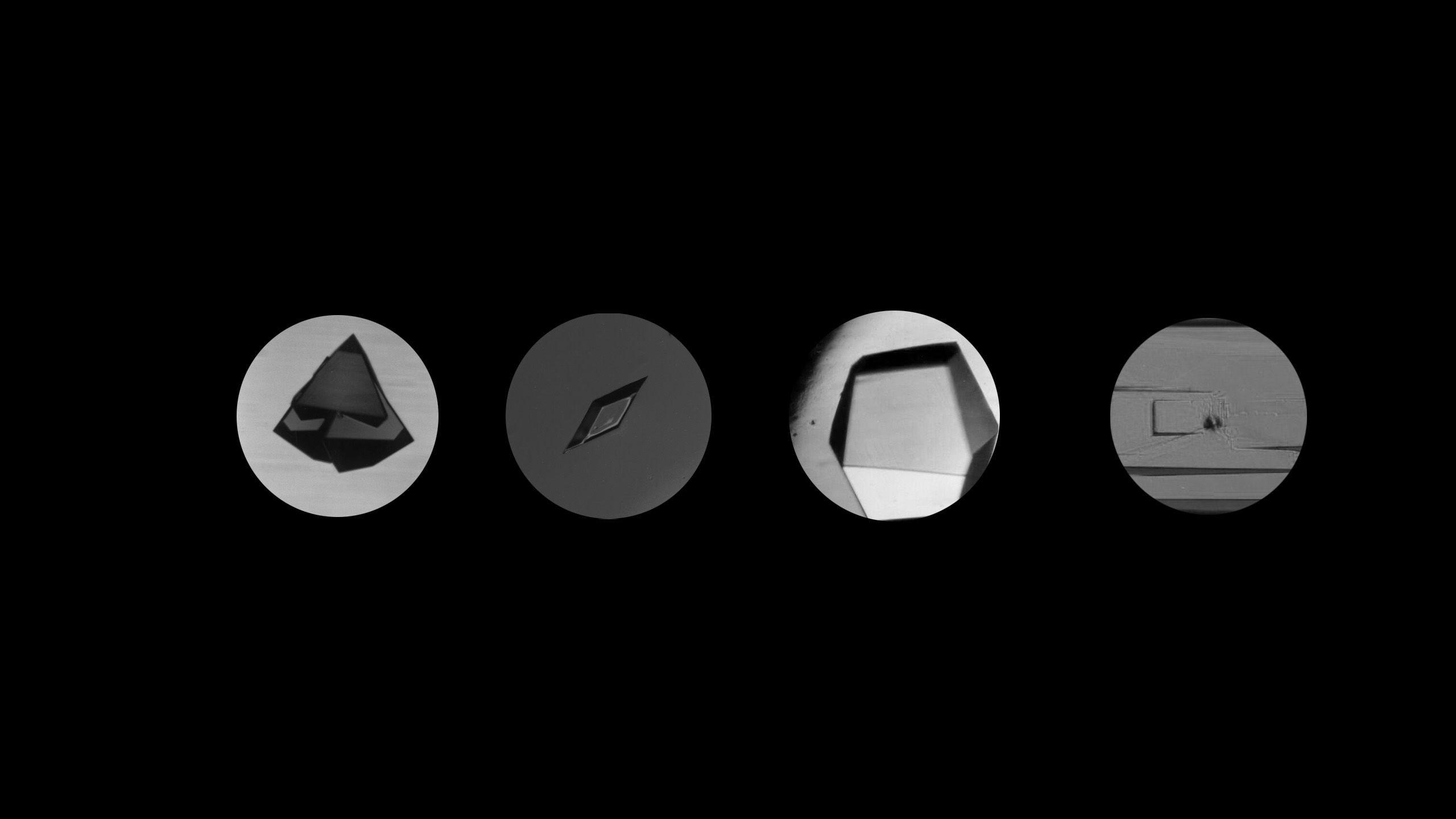
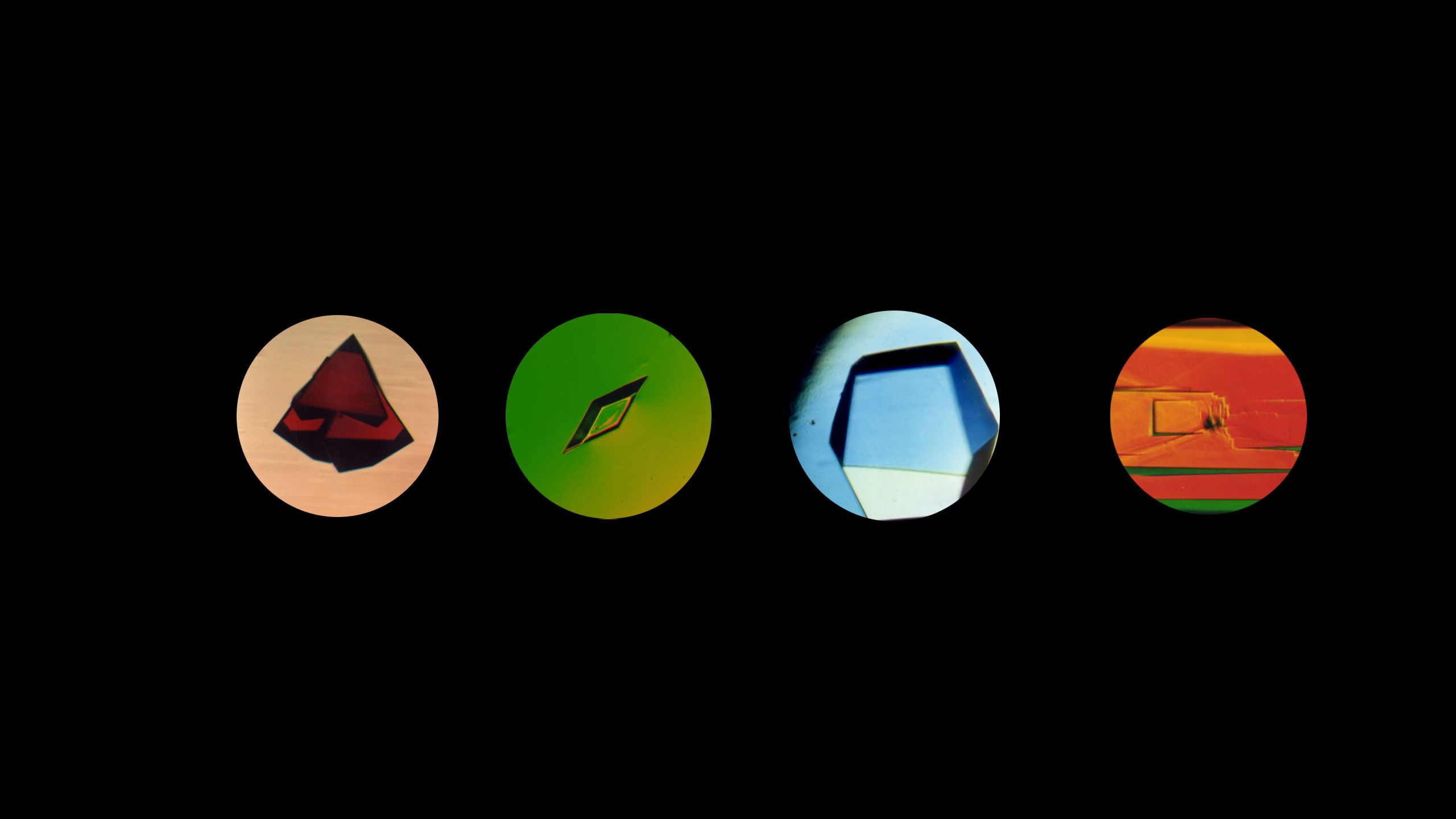

Peter Vekilov, Frank Worley Professor of Chemical and Biomolecular Engineering, published a study on how crystals are formed and how molecules become a part of them.
Peter Vekilov, Frank Worley Professor of Chemical and Biomolecular Engineering, published a study on how crystals are formed and how molecules become a part of them.
So, for historians creating a timeline of crystal fascination and research spanning a million years, mark Jan. 2024 as the time that University of Houston researcher Peter Vekilov, Frank Worley Professor of Chemical and Biomolecular Engineering, published in PNAS an answer to how crystals are formed and how molecules become a part of them.
“For decades crystal growth researchers have dreamt of elucidating the chemical reaction between incoming molecules and the unique sites on a crystal surface that accept them, the kinks,” said Vekilov. The mechanism of that reaction, i.e., the characteristic time scale and length scale, possible intermediates and their stabilities, has remained elusive and subject to speculation for over 60 years.”


The main obstacle to deeper understanding has been the lack of data on how molecules join in, connected to the complicated process of moving from the solution to where they grow.
To unravel the chemical reaction between a molecule that dissolves in liquid (solute) and a kink, Vekilov mobilized two transformational strategies, one using full organic pairs and the second, using four solvents with distinct structures and functions. Working with the molecules, he combined state-of-the-art experimental techniques including time-resolved in situ atomic-force microscopy at near-molecular resolution, x-ray diffraction, absorption spectroscopy and scanning electron microcopy.
That’s when Vekilov made a revolutionary discovery: Incorporation into kinks may occur in two steps divided by an intermediate state and the stability of this middle state is key in how crystals grow. It basically decides how fast or slow the crystals form because it affects how easily things can join in during the process

Vekilov uses the NanoRacer, which uses atomic force microscopy, to scan samples at high speeds and obtain crucial insights into the crystals' molecular structure.
Vekilov uses the NanoRacer, which uses atomic force microscopy, to scan samples at high speeds and obtain crucial insights into the crystals' molecular structure.
“Equally important, this paradigm will guide the search for solvents and additives that stabilize the intermediate state to slow down the growth of, for instance, undesired polymorphs.”
- Peter Vekilov

Vekilov holds a crystal of potassium dihydrogen phosphate (KDP).
Vekilov holds a crystal of potassium dihydrogen phosphate (KDP).
Though the new discoveries don’t date back to Homo Sapien times, they do solve a 40-year-old riddle for Vekilov.
“The notions of an intermediate state and its decisive role in crystal growth refute and replace the dominant idea in the field, brought up by A.A. Chernov, my PhD advisor, that the activation barrier for growth is determined by the solute-solvent interactions in the solution bulk,” he said.
The new paradigm of two step incorporation, mediated by an intermediate state, could help in understanding how small parts in a liquid can influence the detailed shapes of crystals found in nature.
“Equally important, this paradigm will guide the search for solvents and additives that stabilize the intermediate state to slow down the growth of, for instance, undesired polymorphs,” Vekilov said.
Vekilov’s team includes Jeremy Palmer, Ernest J and Barbara M Henley Associate Professor of chemical and biomolecular engineering; former graduate students Rajshree Chakrabarti and Lakshmanji Verma; and Viktor G. Hadjiev, Texas Center for Superconductivity at UH.
