You may know little to nothing about the carbon fiber market, but products produced with carbon fibers are pervasive in your everyday life ... from bicycles and computer hardware to automobiles, apparel and medications.
A University of Houston researcher is working to resolve a major carbon fiber industry pain point that routinely threatens to bring U.S. manufacturing from disparate industries to a temporary but costly halt.
ENGINEERING DYNAMIC SOLUTIONS
Lars Grabow's Research to Bring Chemical Production and Manufacturing Together Could Revolutionize Numerous Chemical Processes
By Nicole Johnson | Photography by Ben Corda

Lars Grabow at the University of Houston received a $2 million award from the U.S. Department of Energy to develop the technology for a catalytic process to produce acrylonitrile in small modular reactors near geographically distributed carbon fiber plants, meaning cheaper products with reduced energy costs.
The bulk of U.S. petrochemical feedstocks are located on the U.S. Gulf Coast, in close proximity to upstream products such as oil and natural gas as well as refineries, reducing the cost of logistics. Additionally, most ports and terminals for import and export are located along the Gulf Coast to facilitate intrastate barge and international vessel movements.
While the location offers many advantages, disruptive weather events like hurricanes, freezes and floods — particularly in recent years — often push Gulf Coast facilities offline. Manufacturers responsible for producing end-products, or derivatives, of these main petrochemicals often see feedstocks stranded during these seasonal force majeure events and their production halted due to a bottleneck hundreds or even thousands of miles away.
This is the story of the U.S. carbon fiber market.
Carbon fiber is a strong but lightweight material used in high-performance products such as sporting equipment, aircrafts and racecars. This ubiquitous material is made from the feedstock acrylonitrile produced primarily on the Gulf Coast, but most carbon fiber manufacturing facilities are located on the East and West Coasts and areas in between.
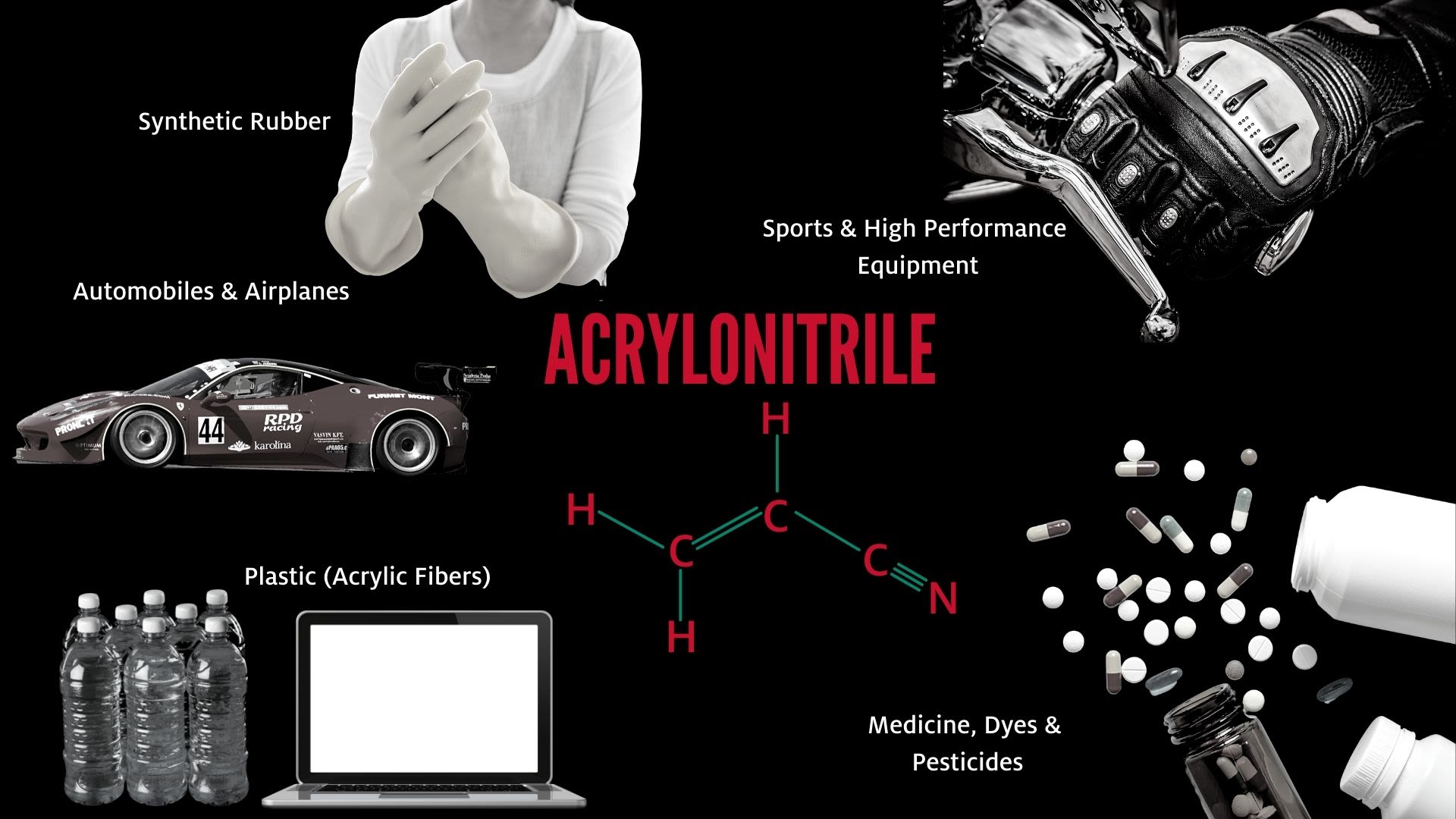
Acrylonitrile is the feedstock used to make carbon fiber, which is used in the manufacturing of an array of products, from vehicles and performance sports equipment to wind turbine blades and electronics.
Acrylonitrile is the feedstock used to make carbon fiber, which is used in the manufacturing of an array of products, from vehicles and performance sports equipment to wind turbine blades and electronics.
The challenge of bringing acrylonitrile (ACN) production closer to carbon fiber production facilities is a question of upstream feedstock access, safety, affordability and catalytic flexibility to optimize acrylonitrile production.
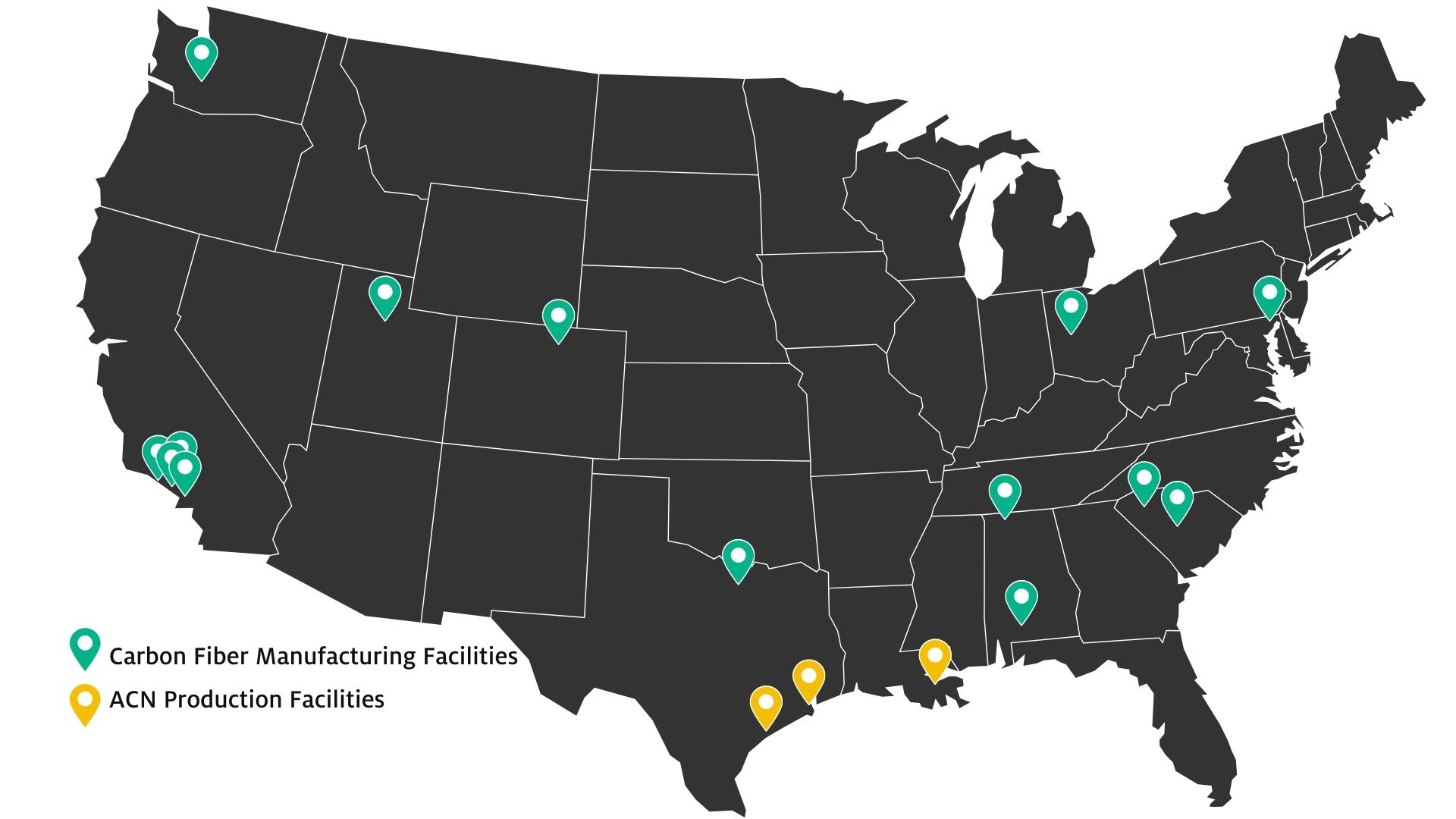
ACN is produced primarily on the Gulf Coast and is the feedstock for carbon fiber, which is manufactured in facilities from several hundred to several thousand of miles away.
ACN is produced primarily on the Gulf Coast and is the feedstock for carbon fiber, which is manufactured in facilities from several hundred to several thousand of miles away.
But a solution may be on the horizon.
Grabow, who serves as Dan Luss Professor at the Cullen College of Engineering and principal investigator, received a $2,091,874 award from the U.S. Department of Energy to resolve this industry pain point by engineering a dynamic process using small reactors near geographically distributed carbon fiber plants that would effectively bring acrylonitrile production onsite. Grabow will work in tandem with team members from Idaho National Laboratory, the University of Virginia, Pacific Northwest National Laboratory and KX2 Development.

Lars Grabow, Dan Luss Professor at the University of Houston Cullen College of Engineering
Lars Grabow, Dan Luss Professor at the University of Houston Cullen College of Engineering
The ingenuity behind how this innovative, ACN-producing reactor works is not dissimilar to how your car works.
“Most people are familiar with how the three-way catalytic converter in a car converts carbon monoxide, nitrogen oxide, and hydrocarbons into carbon dioxide, nitrogen and water. This inspires the research we are doing,” said Grabow.
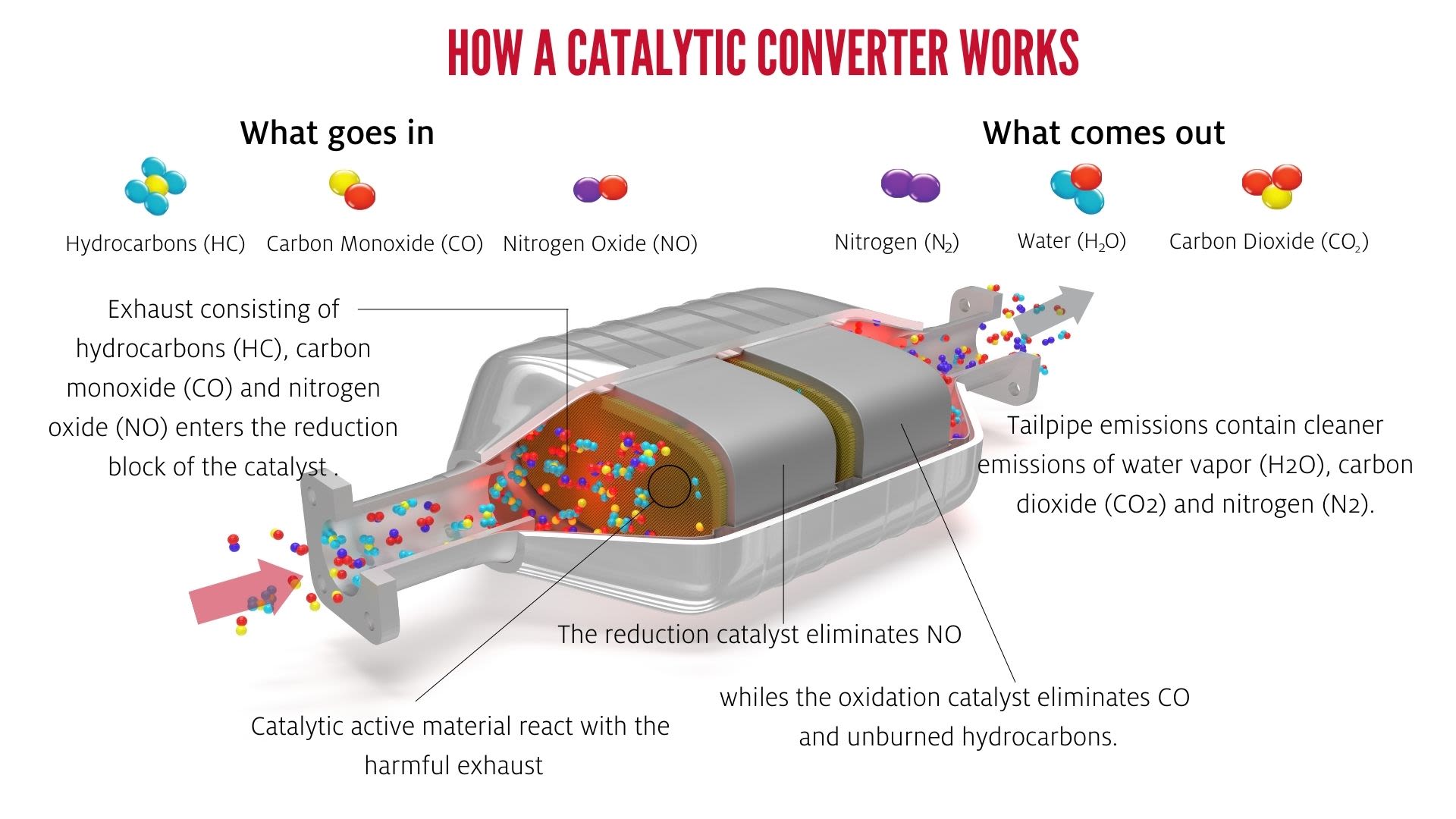
“Car engines are dynamically operated and undergo different driving cycles. To maintain optimal performance of the engine and the three-way catalytic converter under all driving conditions a lambda sensor (oxygen sensor) is used to control and continuously adjust the air to fuel ratio. We have evidence that this externally forced dynamic operation between more fuel-rich versus oxygen-rich states can improve the performance of these smaller reactors.”
This has not been previously done in larger-scale petrochemical units because the concentration of large flow rates cannot be altered quickly, limiting flexibility capabilities. But smaller reactors have more flexibility and shorter response times, such that small pulses of oxidant can be delivered as needed and leveraged to improve the kinetics and performance of the overall process.
“Distributed Chemical Manufacturing, or DCheM for short, is a concept that aims to revolutionize the process industries sector by deploying modular processes and small chemical plants close to the feedstock or to the customer,” said Triantafillos Mountziaris, William A. Brookshire Department Chair and Professor of Chemical & Biomolecular Engineering at the University of Houston.
“This approach can take advantage of geographically scattered feedstocks and provide opportunities for economic development to rural and remote areas. It can also enable on-site production of dangerous chemicals to avoid their transportation and storage costs and risks. The project that Dr. Grabow will pursue is very timely and addresses such a need. If successful, the project may yield significant economic and environmental sustainability benefits. It will also set a new paradigm for the design or retrofitting of additional chemical processes.”

"Transient reaction modulation has the potential to revolutionize numerous chemical processes. Grabow and coworkers selected a very interesting and important problem, and are well-equipped with this new U.S. Department of Energy grant to optimize ACN production and take dynamic catalysis to even greater heights."
The development of small-scale reactors onsite also means improved safety opportunities for ACN production, as the feedstock is a highly explosive chemical that presents high risks during transportation.
On top of strategic geographic access to feedstock and improved safety, the potential of these small-scale modular reactors also points to improved affordability, which speaks volumes for a typically energy-intensive and therefore costly production process for ACN.
“Our process involves periodically switching the oxidation and reduction state of the catalyst surface to speed up the overall catalytic cycle,” said Grabow. “In the first 18 months of funding we are aiming to demonstrate a 20°C reduction in reaction temperature while maintaining identical ACN yield, but there will likely be room for more.”
The production process for ACN using this innovative process can even utilize biomass as a feedstock as opposed to petrochemicals generated from fossil fuels – a step toward improving the energy mix in the energy transition.
But varied feedstocks are not the only flexible factor of this new production process. Improved yield of acrylonitrile versus its poisonous byproducts is another aim of this project, as well as improved lifetime of the catalyst.
“There are indications that by changing the frequency of lean/rich cycling you can change product distribution, and favor selectivity and yield to the desired product,” said Grabow. “We are looking to find the right frequencies that produce the desirable product – acrylonitrile – versus a byproduct, such as hydrogen cyanide, which is a poison.”
The catalyst for acrylonitrile, promoted bismuth molybdate, lasts with regeneration periods about ten years.
“The cycling done to lower the temperature in our dynamic process can also be used to regenerate the catalyst while it is in use,” said Grabow. “This continuous regeneration would eliminate production downtimes due to catalyst deactivation. One of the first steps we take in this project will be to establish the opportunities and limits of dynamic operation.”
The multifaceted benefits of this project would provide a myriad of solutions to several pain points in the carbon fiber market industry, ultimately leading to a cheaper, safer product for the average person.
“Grabow’s U.S. Department of Energy-funded project to develop a cyclic dynamic transient process for acrylonitrile production offers an excellent prospect for making a step-change in the operational performance and efficiency of this industrially important process,” said James Brazdil, professor of chemical and biomolecular engineering at the University of Houston, who previously served as research and development (R&D) manager at INEOS Technologies and Head of the Nitriles Catalyst R&D division.
“The research will provide new fundamental mechanistic insights into the redox chemistry that can apply broadly to the chemical catalysis of heterogeneous selective oxidation. The industrial application of transient kinetics to selective oxidation and ammoxidation processes has been long recognized as offering potential for increased selectivity to valuable industrial products, including acrylonitrile. So, it is with the recent advances in both the equipment and computer capabilities for precise process control, both of which are at the heart of Grabow’s project, that these benefits could now be realized.”
And the chemistry of it all is akin to making your car go. Who’d have thought?
